Lithium Mining
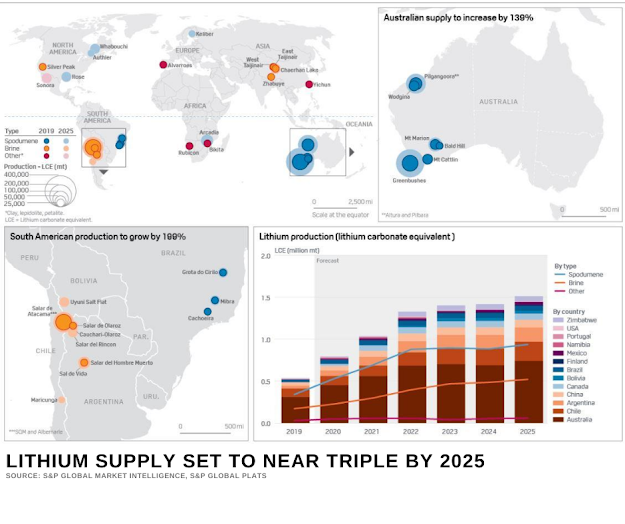
Known as "SALARS" an overwhelming quantity of today’s lithium is extracted from liquid brine reservoirs that are located beneath salt flats, most of which are located in southwestern South America and China. ~ 70%
Well over 100 different minerals contain some amount of lithium, however, only five are actively mined for lithium production (Spodumene, Lepidolite, Petalite, Amblygonite, and Eucryptite). Spodumene is most common mineral. Mineral ore deposits are often richer in lithium content than are salar brines, however, they are costly to access since they must be mined from hard rock formations. Due to the added energy consumption, chemicals, and materials involved in extracting lithium from mineral ore, the process can run twice the cost of brine recovery
- Technical-grade lithium concentrates - low iron content for use in the manufacture of glass, ceramics and heat-proof cookware
- High yielding chemical-grade lithium concentrate - used to produce lithium chemicals which form the basis for manufacture lithium-ion batteries for laptop computers, mobile phones and electric cars.
Chile holds most of the world's “economically extractable” lithium reserves, and its Salar de Atacama hosts approximately 37 percent of the world’s lithium reserve base.
There is a significant activity in Lithium mining to meet the future requirement
Applications if Lithium
Lithium is widely used in heat transfer applications. The following are the other application areas of lithium:
- To make special glasses and ceramics
- In electrical and electronic components
- To make lubricating greases
- As a flux for welding or soldering.
- Lithium fluoride - in specialist optics for IR, UV and vacuum UV applications
- Lithium niobate - in non-linear optics applications
- Lithium chloride and lithium bromide - used as desiccants for gas streams.
One of the major application is its use in combination with other metals such as Nickle (Ni); Cobalt (Co); Iron (Fe); Manganese (Mn) in making cathode for all kinds of batteries. Lithium is essential part of batteries and hence the name Lithium-ion (Li-Ion) batteries. Typically these 4 key metals with Lithium constitute ~ 55% of Li-ion batteries.
There are 5 types of Li-ion Batteries each has its own advantages and disadvantages and their unique use in various applications.
- Lithium Cobalt Oxide - LCO - LiCoO2
- Lithium Nickle Manganese Cobalt Oxide - NMC - LiNiMnCoO2
- Lithium Manganese Oxide - LMO - LiMn2O2
- Lithium Nickle Aluminum Cobalt Oxide - NCA - LiNiCoAlO2
- Lithium Iron Phosphate - LFP - LiFePO4
As a example LCO is used in 2W; 3W; EV applications and light storage batteries while NMC; LMO; NCA finds applications in 4W EV-batteries. lastly LCO finds its application in cellphones and laptops.
There are certain advantages and disadvantages of Li-Ion Batteries
Advantages
- High energy density - potential for yet higher capacities.
- Does not need prolonged priming when new. One regular charge is all that's needed.
- Relatively low self-discharge - self-discharge is less than half that of nickel-based batteries.
- Low Maintenance - no periodic discharge is needed; there is no memory.
- Specialty cells can provide very high current to applications such as power tools.
Limitations
- Requires protection circuit to maintain voltage and current within safe limits.
- Subject to aging, even if not in use - storage in a cool place at 40% charge reduces the aging effect.
- Transportation restrictions - shipment of larger quantities may be subject to regulatory control. This restriction does not apply to personal carry-on batteries.
- Expensive to manufacture - about 40 percent higher in cost than nickel-cadmium.
- Not fully mature - metals and chemicals are changing on a continuing basis
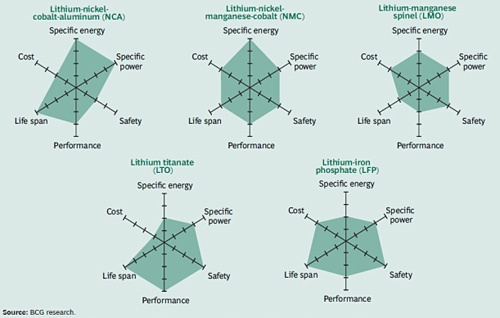
- Safety is one of the most important aspects when choosing a battery for the EV
- Life Span reflects cycle count and longevity. Most EV batteries are guaranteed for 8–10 years or 160,000 km (100,000 miles). Capacity loss through aging is a challenge, especially in hot climates. To compensate for capacity loss, EV manufacturers increase the size of the batteries to allow for some degradation within the guaranteed service life.
- Performance reflects the condition of the battery when driving the EV in blistering summer heat and freezing temperatures. Unlike an IC engine that works over a large temperature range, batteries are sensitive to cold and heat and require some climate control.
- Specific energy demonstrates how much energy a battery can hold in weight, which reflects the driving range. It is sobering to realize that in terms of output per weight, a battery generates only one percent the energy of fossil fuel. One liter of gasoline (1kg) produces roughly 12kW of energy, whereas a 1kg battery delivers about 120 watts. We must keep in mind that the electric motor is better than 90 percent efficient while the IC engine comes in at only about 30 percent. In spite of this difference, the energy storage capability of a battery will need to double and quadruple before it can compete head-to-head with the IC engine.
- Specific power demonstrates acceleration, and most EV batteries respond well. An electric motor with the same horsepower has a better torque ratio than an IC engine.
- Cost presents a major drawback. There is no assurance that the battery’s target price of $250–400 per kWh, which BCG predicts, can be met. The mandated protection circuits for safety, battery managements for status, climate control for longevity and the 8–10-year warranty add to this challenge. The price of the battery alone amounts to the value of a vehicle with IC engine, essentially doubling the price of the EV.
Lithium Content Per Batteries
According to battery university "A 2Ah 18650 Li-ion cell has 0.6 grams of lithium content."So, for Tesla's 3250 mAh batteries, the lithium content would be
(0.6/2)*3.250 = 0.975 g Li.
Now, with an operating voltage of 4.2V, the lithium content would be:
0.975/(3.250*4.2) = 0.0714 g/Wh = 0.0714 kg/kWh.



Comments